Imagine having your personality change drastically and not being able to recognize why you’re so different. You look the same, and you talk to the same people, but for some reason, you feel unusual. Something about the way you think and feel is becoming unsettling and irritating. You seem to realize that nearly everything you think about is on the tip of your tongue. You feel like your mind is in chaos and your brain is scrambling to remember what happened. You realize that you’re starting to lose track of time, and you’re beginning to forget not only objects, places, and words, but also people. You get diagnosed with Alzheimer’s, but unfortunately, you can’t even remember that.
Causes and History of Alzheimer’s
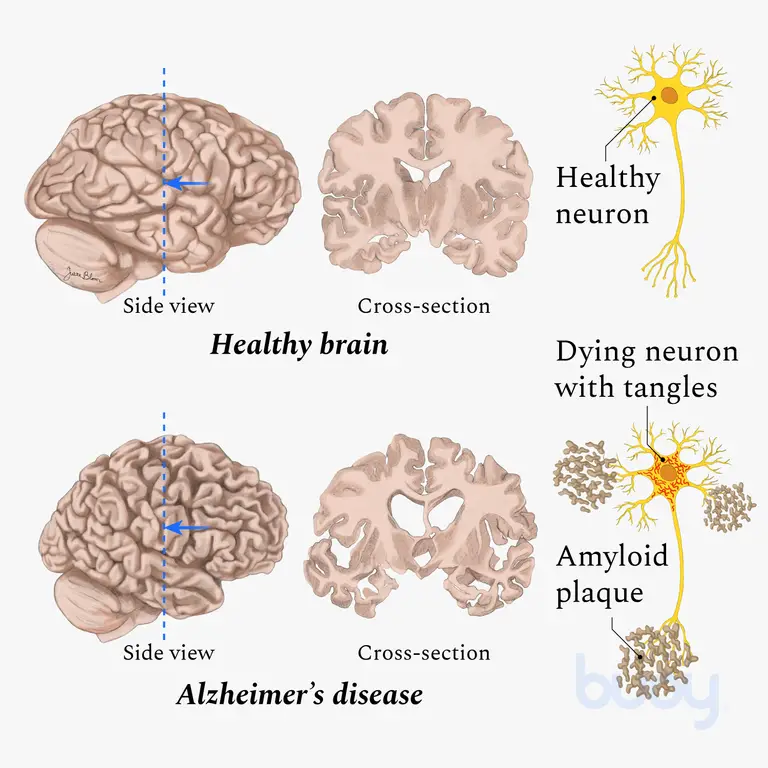
Nearly 6.5 million Americans have this heartbreaking disease, and most of them are 65 or older. There isn’t a single root cause for Alzheimer’s, but it is thought to be a combination of genetic, aging, lifestyle, and environmental factors. Certain genes/alleles are considered to be a hereditary risk factor. The molecular basis for Alzheimer’s is likely due to buildup of beta amyloid plaques and tau neurofibrillary tangles in brain tissues. This excessive amount of protein might be the reason that memory and thinking deteriorate with Alzheimer’s. Brain scans indicate that there is significant neurodegeneration and shrinking of the hippocampus and cerebral cortex.

In addition, there is a wide spectrum of symptoms, but common ones comprise dementia, personality changes, and difficulty with problem-solving and decision making. There may be sleep, speech, behavior, and cognitive issues. Long-term problems include significant memory losses, such as forgetting how to swallow, how to move, or when to eat.
Introducing Lecanemab
It’s agonizing to see a loved one go through Alzheimer’s. A cure for this overwhelming disease is what everyone wants; however, there is still a significant amount of research that needs to be done. A new drug called Lecanemab, developed by Biogen and Eisai, is in Phase 3 clinical trials and is considered to be a breakthrough drug for preventing Alzheimer’s, but many disagree. Lecanemab works by releasing monoclonal antibodies that propagate defense mechanisms against the beta amyloid protein buildup in the brain. Even though high levels of this protein are associated with Alzheimer’s, there isn’t sufficient evidence to support that this specific buildup causes Alzheimer’s or that reducing the buildup will significantly help. Many researchers think that it may be a combination of both tau and beta proteins, along with other factors. Nonetheless, researchers are optimistic that Lecanemab may actually reduce both beta and tau, slowing the progression of the disease.
The Obstacles
Despite its benefits, Lecanemab does come with some caveats. This drug doesn’t prove to reverse any brain damage, and seems to be beneficial for only those who have early-stage or mild Alzheimer’s. It can only slow the advancement of the disease, and it can’t really treat other forms of dementia. Also, this drug still needs to be approved by the FDA, and the prices and insurance policies are still unclear. Another recently approved Alzheimer’s drug, Aduhelm, was initially priced at around $56,000 per year. Costs may pose a significant barrier for these types of drugs since Lecanemab needs to be administered for life with infusion therapy, so the better/longer the drug works, the higher the value.
What Current Tests Show
Lecanemab’s Phase 3 clinical trial lasted for only 18 months, which showed decelerated cognitive decline, but it’s difficult to predict if this could last in the long run. Alzheimer’s, similar to other chronic diseases like cancer, is likely different for each patient. Therapies might only treat certain forms of the disease. Recent PET scan developments use dyes to identify buildup of tau and beta proteins, but it’s still difficult to measure cognitive decline, which adds to the challenge of diagnosing and dosing.
Another obstacle includes not being able to participate in other, potentially better, drug trials if you are already participating in this one. Biogen and Eisai announced that this trial was also exploring how Lecanemab can impact people who don’t have dementia but have varied levels of beta amyloid. In addition, the trial is investigating how using both Lecanemab and another drug that targets tau protein may influence the progression of Alzheimer’s.
Many people are willing to try anything to restrain Alzheimer’s, but there are some who aren’t able to afford it at all. The drug itself might be expensive, but treatment also requires mental, physical, and emotional strength and stability. Witnessing the disease’s destructive path is burdensome, but caring for people who have it may be even more tolling. It requires constant care, attention, and motivation. Would people really be willing to wage so much on a drug that might prove to be minimally effective?


